132
Views & Citations10
Likes & Shares
EXPERIMENTAL METHODS
Materials and Reagents
Made from California Estate Extra Virgin Olive Oil, Olive oil was produced in St. Lauderdale (F.L., USA) and purchased from a supermarket (Stop and Shop, New City, NY). The Emulsifying Wax NF, Non-GMO Premium Quality Polysorbate 60/ Pola wax 80 oz / 5 Pound was obtained at Plant Guru (Plainfield, NJ). Reverse Osmosis filtering water was purchased from Poland Water Supply (Palisades Park, NJ). Other vital ingredients, i.e., maltitol, were bought from Sigma Aldrich (St. Louis, MO). Further, Sodium Bicarbonate from Bulk Supplements ScienceLab.com Inc (Houston, TX), Vitamin D (cholecalciferol-D), Sodium Dodecyl Sulfate, Polysorbate 80 from the Spectrum Chemical Corp (New Brunswick, NJ). Hydrogen peroxide from Wall Green Pharmacy Inc., Thera Breath Oral Care Probiotics Citrus from Amazon.com, and Multiple Food Color (Net 1 Fl Oz 29 mL) from Earth Health, Inc. (Hunt Valley, MD). DMSO was obtained from Sigma Aldrich (St. Louis, MO). Three commercial toothpastes were obtained and used to examine our formulation, comparing their characteristics for our intraoral vitamin D toothpaste. The Colgate Toothpaste Total S.F. was created for antigingivitic, anticavity, and non-sensitivity toothpaste with stannous fluoride. Secondly, the Crest brand toothpaste, formulated for tartar protection (Sensitive paste) with fluoride anticavity ability, was bought from the local marts (CVS, Orangeburg, NY). The third brand was Meich PUSH toothpaste (Broadway Drug Store, NJ).
Emulsion Formulation
The formulation and testing methods were published previously for other types of penetration enhancers [30]. Glassware and experimental supplies were collected. Two 300 mL beakers were gathered, and the beakers were written as aqueous and oily phases on the surface. One hundred twenty milliliters of distilled water were poured into the aqueous beaker, and 20 mL of olive oil, 30 mL of polysorbate 80, and 45g of emulsifying wax were measured and poured into the oily marked beaker. Then, the beakers were moved onto a stainless-steel container with water with a jacket and heated to a yellowish melting solution at approximately 70 degrees Celsius. After confirming a complete melting in the oily phase container, the contents of the water-phase beaker were combined into a mixing ball crystal glass cup. The ingredients in the crystal glass cup were then brought to an electric stirring of 1500 ~ 2000 rpm, adjusting its intensity according to the thickness of the mixture for approximately 60 min.
Intraoral Formulation for Vitamin D Delivery (IOVD)
While the mixture was agitated, other liquids were measured out, and granular chemicals were taken out into a 500 mL beaker. The contents as seen in Table 1 below were collected and ground manually into powder form using a plastic scoop. Those ingredients were 5.0 g sodium bicarbonate, 4.0 g Silica, and 3 g of Vitamin D. The beaker was brought into the stirrer approximately for sixty minutes and combined with the contents in a round-bottom glass cup.

After that, 10.0 mL of glycerin and 6.0 mL of hydrogen peroxide were added and mixed. Further, the thoroughly blended final product was divided into two groups, where the first portion was formulated with 3.50 mL DMSO.
Quality Evaluation of Toothpaste
Stock solution preparations
Brought in 100 mL distilled water, the 4.0 g toothpaste was weighed out into a 300 ml container as in Figure 1. Subsequently, the container with the toothpaste was utterly dispersed by a stirring bar on an electric magnetic stirrer. The other stock solutions were created with identical procedures for comparison groups. Then, a magnetic bar was dropped into the containers, which then brought on a magnetic stirrer and stirred for approximately 20 min to attain homogeneous dissolution. The stock solutions were consumed for pH evaluation and foaming capability evaluations. The stock solutions were placed in the cold refrigerator (4°C) prior to being used.

Abrasiveness degree Score evaluation
Approximately 2.5 cm of toothpaste was obtained from its tube and placed onto a piece of waxed paper. Each stripe of toothpaste was smeared with the middle finger for the length of approximately 5.0 cm to closely observe the edges of rough and sharp-edged portions with soft and runny edge shapes. The degree of the sharp-edged profile was carefully observed and scored from 0 - 5; 0 for smooth edges while 5 for rough grainy edges.
Scratchiness degree evaluation
The scratchiness evaluation was carried out as follows: 2,0 g of each toothpaste was squeezed out from the tube on a sterilized, individualized wrapped petri dish, and 200 ul distilled water was dropped over the sample using an Eppendorf pipette as in Figure 2. The toothpaste on the petri dish was stroked back and forth using a sterilized cotton ball, 5.0 centimeters, approximately thirty times. Then, the toothpaste was utterly washed out from the petri dish using running tap water and thoroughly dried with a paper towel. The scratchy surface created on the petri dish was then carefully observed under a digital microscope to grade the degrees of scratches on the surface. The degree of scratches was then graded on a score, from 0 when no scratches existed to 5 when highly outstanding scratches were over the surface. The evaluation was carried out five times for each toothpaste brand.
Spreadability evaluation
A toothpaste of 2.0g was placed on the electric balance to weigh out by pushing from the tube and distributed on the plexiglass panel. And then, an additional plastic plate was prepared to cover the toothpaste. Then, a 500g standard weight was used to press the toothpaste on the plexiglass plate for 20.0 min as in Figure 3. Immediately after removing the weight standard, the diagonal and its vertical lengths were evaluated with a ruler. Its size was measured as mean and standard deviation.
 4
4
pH Measurement
The Thermo Scientific A111 pH meter was calibrated first regarding pH 4.0 and 10.0 according to the vendor's user guidelines explained. A 30 mL toothpaste stock solution was acquired and brought into 150 ml beakers before the pH measurement. After setting up all necessary preparations, the pH was read after the pH electrode was slowly inserted into the stock solution with the confirmation of room temperature.
Foam-generating ability evaluation
Thirty millimeters of the stock solutions of each toothpaste prepared in advance were poured into 200 ml graduated cylinders. The height of the stock solution was marked on the surface. The cylinder was subsequently moved back and forth 20 times vigorously with its mouth closed with a piece of thin plastic wrap and pressed down with a thumb finger so as not to spill as in Figure 4. The graduated cylinder was then put on a flat, balanced table surface, and the foam's length in the cylinder was evaluated.

Tooth cleaning ability evaluation
The toothpaste’s cleaning ability was examined for the toothpaste's feasibility to rid the red food dye colored on the skin of eggs. The procedure was performed with four eggs boiled in distilled water with red food coloring dye to paint the surface as red for 25 min and left them to cool down at room temperature for 20 min. After that, a mid-line was marked on the surface of the eggshells, dividing it in half with a permanent marker. To mimic the typical toothbrushing manner, the toothbrush was made wet with water, and removed any surplus water. On one side of each colored egg, 25 reciprocating toothbrush movements of 6.0 cm in length were repeated for each of the toothpastes of the comparative groups as in Figure 5. The magnitude of the region at which color was seen to fade out was visually outlined and estimated for its shortest and longest length as centimeters to quantify the area of each cleaned-out contour.

Antibacterial strength evaluation with the ring of inhibition
A set of bacterial cultures were obtained from Carolina Biological Supply. The strains were identified with the Gram-stain method, since it was the first step in defining a bacterial family. First, the pigmented bacteria strains were Micrococcus luteus (MIC), as yellow as MIC, Rhodococcus rhodochrous (RHO), as pink as RHO, Sarcina aurantiaca (SAR), as orange-yellow as SAR, and Serratia marcescens (SER) D1, as red as SER for the differentiating methods of procedures as in Figure 6. Four Petri dishes were divided into four quarters: 1, 2, 3, and 4, and labeled accordingly. The bacteria were inoculated onto the Petri dishes already coated with nutrient agar with an inoculation wire loop. And the stock solutions of the four kinds of toothpaste were soaked into the circular paper disks that were prepared in advance. The disk was placed with the toothpaste applied on the four areas as soaked and written: 1 - IOVD, 2 - Colgate, 3 - Crest, and 4 - Push toothpaste, as found in Figure 7 below. The bacterial culturing procedures were performed in a Heratum Incubator (Thermo Scientific, Waltham, MA) for 72 h.


Indirect skin transport evaluation with Lumbricus terrestris
Sixteen L. terrestris were grouped into four groups in 250 ml beakers with labels on the surface after measuring their body weight. Subsequently, 3.0 gms toothpaste was applied around their skin. Their body was weighed again immediately after 1.0 h. The body weight difference was evaluated and reported as weight change%.
Evaluation of transepithelial electrical (TEER) values
Many drug delivery groups in the pharmaceutical company usually evaluate the TEER parameters to measure the movement of natural and synthetic elements of interest. The opening of tight junctions might be momentarily and almost instantaneously without adverse effects under the presence of penetration enhancers [31]. This transport can be evaluated by monitoring the transepithelial electrical resistance (TEER) that relates to passive paracellular transport of molecules, whereby reduced resistance to an electrical current increases paracellular transport of an inert compound into the opposite side, indicating the opening of cell junctions. For the study, a dissolved solution was prepared with the toothpaste of 60.0 g mixed with 200 mL of purified water with a reverse osmosis (R.O.) system to create a 20% solution for each toothpaste. The 30-centimeter enamel-coated 10.0-gauge copper wires created two electrodes for positive and negative poles after the coated insulation layer was removed with a typical electrical stripper plier. Both tips of the wire formed a ball shape with soldering, leading to softening their insertion into the L. terrestris through its mouth. Data I-245 Data Acquisition System (Dataq Instrument, Ohio) was employed to acquire the reducing resistance across the epithelial layer by the effect of toothpaste solutions. A 9.0 V dry battery was wired during the study to upgrade the electrical potential. Post-completing the procedures in toothpaste solutions and initiating the data acquisition, the earthworm was washed, and its body weight weighed on an electrical balance and brought into a plastic beaker filled with 5% ethyl alcohol for anesthetization. After confirming the unconsciousness, about 8 min, it was taken into the test tube and poured with the toothpaste solution up to 80% of the height of the test tube, 18x150 mm. Subsequently, the positive electrode was cautiously pushed into the mouth of the animal approximately up to the middle length posterior to the clitellum. The other tip of the positive electrode was wired to the matrix and P.C. computer with the positive button of a 9V battery. On the other hand, the negative electrode was just placed into the testing solution of 30 ml wired to the system and the negative tip of the battery. Data were automatically saved while monitoring the data acquisition display mode. The data was played back using the waveform browser software (Akron, OH) after the experiment and transported into M.S. Excel. The data was summarized, graphed, and analyzed in M.S. Excel with trendline functionality as planned in advance.
Data analysis and summary
Mean and standard deviation were the summarized methods first. Some qualitative data were graded into a numeric conversion, supporting quantitative comparison between groups. A student t-test was carried out if needed (P<0.05). The TEER slopes were estimated in the spreadsheet in M.S. Excel with the regression analysis.
RESULTS AND DISCUSSIONS
Toothpaste Creation Procedures
The toothpaste for intraoral vitamin D delivery was prepared from an emulsion base preparation created with distilled water, olive oil and emulsifying wax. The formulation was completed after thoroughly blending toothpaste ingredients manually. Any granular compositions were homogeneously broken down to powdery in a crystal jar with a large plastic spatula and mixed for 30 min to be like a form of sticky dough. The toothpaste did not have any coloring or flavoring chemicals supported. To increase the user's attraction to toothpaste, decorating a trace of mint fragrance or green dye could be an additional decoration. Other classical toothpaste ingredients, i.e., abrasives, humectants, and binders, were mixed sequentially. To formulate a user-friendly toothpaste, its viscosity and texture were controlled by balancing the optimal amount for each composition.
Abrasive Ability Examination
Tooth abrasion is a primary toothpaste interest for general consumers. The key degrading characteristic of toothpaste is its abrasiveness. Therefore, trust evaluation and comparison with commercial brand toothpaste are essential. Comprehending the abrasiveness of toothpaste is a must. Some scientists employed a radiometric method for an abrasiveness measurement in silica and calcium carbonate samples for an abrasive in a dentifrice to help in a fantastic choice of materials by dentifrice manufacturers [32]. Patients sometimes like the rough feeling of abrasive particles from toothpaste. Figure 7 shows that all three other toothpastes did not find abrasive findings. However, our toothpaste presented the feeling of particles that were proven with middle fingers. Our vitamin D toothpaste (IOVD) showed a more significant mean abrasive ability score, in contrast to that of other commercial products, and no statistical difference with the Push toothpaste (P<0.05), as seen in Figure 7.
Petri Dish Scratchy Evaluation
A scratchy test was carried out with a Petri dish. This quality evaluation investigated for the toothpaste to clear off the plaque deposits effectively from the tooth's surface. If the scratch was not visually recognizable enough, the toothpaste should still have high feasibility, not removing some food debris. However, when the scratchy ability is too excessive, the tooth's surface enamel might be worn out easily. An optimal scratchy ability might be examined when creating any new toothpaste. The IOVD was demonstrated to be compatible with other commercial products based on our petri dish scratchy evaluation (P<0.05), as given in Figure 8.

Spreadability Evaluation
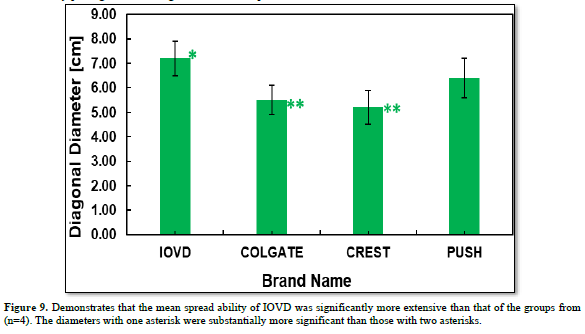
The spreadable ability evaluation was performed to examine how large the diagonal length of the spread toothpaste could be created by placing identical weights over the toothpaste. The diagonal diameter of the spread might be a significant indicator to estimate because the larger spread could enhance vitamin D transport. Our IOVD toothpaste showed the largest mean diagonal spread ability, as in Figure 9.

Toothpaste pH Evaluation
Our teeth' enamel triggers the loss of minerals when the teeth are left at a pH level lower than approximately 5.0. Therefore, the toothpaste pH should be carefully evaluated and controlled accordingly. Using toothpaste with fluoride should be an excellent choice for enamel protection. Daily and long-term use of toothpaste with low pH might melt down the tooth surface. It has been recommended that the pH might be from an acidic 5.76 to a basic, safer side of 9.68 [33]. Given in Figure 10, our IOVD toothpaste pH was as basic as pH 7.14, which is a healthy and safe pH for protecting the enamel layer without harmful effects on the epithelial mucosa of oral cavity.

Foaming Ability Evaluation
Various toothpaste is formulated to increase the hygiene of dental systems using the foaming capability. Toothpaste could generate a soothing feeling in the mouth while brushing. Foam generation enhances its function with better sensations. Evaluating the foaming ability and foam consistency are essential procedures in manufacturing toothpaste below demonstrates that the mean foam length of IOVD was only statistically greater than that of the Push brand. No statistical difference in foaming ability existed from that of Colgate and Crest, as plotted in Figure 11.

Cleaning Capability Examination
Toothpaste should be formulated to remove plaques of the teeth more efficiently and effectively for oral health protection from decay. The fundamental but essential characteristics should be kept alive in their functions because most consumers ask the requirement to hold their teeth with stainless and potentially no harmful bacterial growth. Even though other procedures for monitoring cleanliness are documented [34], the cleaning ability should be verified for any reason. While measuring the cleaning ability with the eggs colored, it appeared difficult to evaluate the cleaning ability of the toothpaste with an apparent definition. Still not for sure, there were improved differentiations that some definition of the discolored region by brushing activities on the surface of the dyed egg was scientifically reasonable. As seen below in Figure 12, the cleaning capability presented by the contour of the exposed regions demonstrated that our toothpaste IOVD was similar to that of other commercial products.

Antibacterial Strength Evaluation
The four disks wetted in the different types of toothpaste were brought on the surface of the agar layer that was inoculated in advance with four strains of bacteria, as mentioned in the Method section. The diameters of the inhibition ring at the divided sections were measured after 72 h of incubation and summarized as the mean value for minimizing any errors in the measurement. The data showed that the antibacterial strength of IOVD was confirmed compared to other brands of toothpaste, as seen in the MIC and RHO strains group. Figure 13 plotted our summarized data on the diameter of the inhibition ring. It was estimated that IOVD has the largest radius among the groups, which showed that IOVD could be able to remove unhealthy bacteria inside the oral cavity.

Mass Transport Study with Earthworm's Skin
It might be called a type of universal law that any object moves from high to low concentrations, which is the principal expression of Fick’s diffusion law. When a concentration gradient of particles is created across the skin, the osmotic pressure must be exerted, and materials inside the skin should be pulled out. If the osmotic press is established, penetration enhancers such as polysorbate 80 and DMSO should increase any transport capability. Sixteen earthworms were grouped into four containers and brought into 300 ml containers with corresponding marks on the glass surface after weighing their body. Finally, 2.0 gms of each toothpaste sample was applied around their skin and held at room temperature for 30 min. Then, their body weight was measured repeatedly. The change in body weight was evaluated and its unit was calculated to be weight change%, as seen in Figure 14.

Material Transport Estimation with TEER Values Across the Skin
Transepithelial electrical resistance (TEER) is a widely recognized indirect quantitative method to evaluate transport through tight junctions in cell culture of epithelial monolayers for drug transport research [35]. TEER values' underlying mechanisms might not be exactly the same as this study's method. The application to the earthworm's skin could be appropriate because the L. terrestris skin is epithelial tissue that is not entirely different from other animals. Our preliminary study for assessing transport capability found that the slope of the TEER value from a regression line had an inverse relationship to the concentration of penetration enhancers. Figure 15 demonstrated that the TEER slope was significantly greater in the IOVD group than in other groups. The data demonstrated that the IOVD toothpaste had a high feasibility of delivering the vitamin into the body of L. terrestris. Study limitations were placed on the fact that no human study was performed.

CONCLUSION
Therapeutic toothpaste with new concepts has developed; a vitamin D deliverable toothpaste has been formulated with DMSO and polysorbate 80 penetration enhancers. The purpose of our study was to formulate a toothpaste that could support the vitamin D transport into the bloodstream using blood vessels in the oral cavity to alleviate the inadequate intake of vitamin D. In conclusion, our formulation was comparable, or better, in various toothpaste characteristic tests compared with commercial toothpaste. TEER measurement confirmed the feasibility of vitamin D transport through oral cavity administration using a toothpaste formulation. Vitamin D should be delivered into the bloodstream by daily brushing our teeth. However, more studies should be executed to clarify various aspects of practical applications.
- Tobias DK, Luttmann-Gibson H, Mora S, Danik J, Bubes V, et al. (2023) Association of body weight with response to vitamin D supplementation and metabolism. JAMA Newt Open 6(1): e2250681.
- European Food Safety Authority (2006) Tolerable upper intake levels for vitamins and minerals. Scientific Committee on Foo, Scientific Panel on Dietetic Products, Nutrition and Allergies, European Food Safety Authority, ISBN: 92-9199-014-0, 2006.
- Aranow C (2011) Vitamin D and the immune system. J Investig Med 59(6): 881-886.
- Moretti R, Morelli ME, Caruso P (2018) Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact. Int J Mol Sci 19(8): 2245.
- Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ (2010) Vitamin D: Metabolism. Endocrinol Metab Clin North Am 39(2): 243-253.
- Nair R, Maseeh A (2012) Vitamin D: The "sunshine" vitamin. J Pharmacol Pharmacother 3(2): 118-126.
- USDA (2020) Dietary Guidelines for Americans; 2020 - 2025, U.S. Department of Agriculture and U.S. Department of Health and Human Services, DietaryGuidelines.gov.
- Laird E, Ward M, McSorley E, Strain JJ, Wallace J (2010) Vitamin D and bone health: Potential mechanisms. Nutrients 2(7): 693-724.
- Office of the Surgeon General (US) (2004) Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (M.D.): Office of the Surgeon General (US); 3, Diseases of Bone. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK45506/
- Yıldırım M, Saral S, Mercantepe T, İskender H, Tümkaya L, et al. (2020) White Tea Reduced Bone Loss by Suppressing the TRAP/CTX Pathway in Ovariectomy-Induced Osteoporosis Model Rats. Cells Tissue Organs 209(1): 64-74.
- Wen H, Jung H, Li X (2015) Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J 17(6): 1327-1340.
- Vinarov Z, Abdallah M, Agundez JAG, Allegaert K, Basit AW, et al. (2021) Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur J Pharm Sci 162: 105812.
- Fallingborg J (1999) Intraluminal pH of the human gastrointestinal tract. Dan Med Bull 46(3): 183-196.
- Minalyan A, Gabrielyan L, Scott D, Jacobs J, Pisegna JR (2017) The Gastric and Intestinal Microbiome: Role of Proton Pump Inhibitors. Curr Gastroenterol Rep 19(8): 42.
- Dahlgren D, Lennernäs H (2019) Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Pharmaceutics 11(8): 411.
- Lam JKW, Cheung CCK, Chow MYT, Harrop E, Lapwood S, et al. (2020) Transmucosal drug administration as an alternative route in palliative and end-of-life care during the COVID-19 pandemic. Adv Drug Deliv Rev 160: 234-243.
- Narang N, Sharma J (2011) Sublingual mucosa as a route for systemic drug delivery. Int J Pharm Pharm Sci 3(Supp 2): 18-22.
- Valshall AP, Earekar AB, Saudagar RB (2015) Review article on sublingual route drug delivery system. World J Pharm Res 4(6): 503-513.
- Bartlett JA, van der Voort Maarschalk K (2012) Understanding the oral mucosal absorption and resulting clinical pharmacokinetics of asenapine. AAPS PharmSciTech 13(4): 1110-1115.
- Bikle DD, Feingold KR, Anawalt B, Blackman MR, Boyce A, et al. (2000) Vitamin D: Production, Metabolism, and Mechanisms of Action. (Updated 2021 Dec 31). In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext (Internet). South Dartmouth (M.A.): MDText.com, Inc.; 2000-. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK278935/
- Sawaya RA, Jaffe J, Friedenberg L, Friedenberg FK (2012) Vitamin, mineral, and drug absorption following bariatric surgery. Curr Drug Metab 13(9): 1345-1355.
- Pandey A, Mittal A, Chauhan N, Alam S (2014) Role of Surfactants as Penetration Enhancer in Transdermal Drug Delivery System. J Mol Pharm Org Process Res 2: 113.
- Hianik T (2007) Structure and physical properties of bio membranes and model membranes. Acta Phys Slovaca 56(6): 687-805.
- Haque T, Talukder MMU (2018) Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv Pharm Bull 8(2): 169-179.
- Akhtar N, Rehman MU, Khan HMS, Rasool F, Saeed T, et al. (2011) Penetration enhancing effect of polysorbate 20 and 80 on the in vitro percutaneous absorption of L-ascorbic acid. Trop J Pharm Res 10(3): 281-288.
- Williams A, Brian B (2004) Penetration Enhancers. Adv Drug Deliv Rev 56: 603-618.
- Moskot M, Jakóbkiewicz-Banecka J, Kloska A, Piotrowska E, Narajczyk M, et al. (2019) The Role of Dimethyl Sulfoxide (DMSO) in Gene Expression Modulation and Glycosaminoglycan Metabolism in Lysosomal Storage Disorders on an Example of Mucopolysaccharidosis. Int J Mol Sci 20(2): 304.
- Pereira R, Silva SG, Pinheiro MB, Reis S, do Vale ML (2021) Current Status of Amino Acid-Based Permeation Enhancers in Transdermal Drug Delivery. Membranes 11(5): 343.
- Gironi B, Kahveci Z, McGill B, Lechner BD, Pagliara S, et al. (2020) Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys J 119(2): 274-286.
- Naree L, Lee J (2023) Investigating a vitamin D delivery toothpaste using a penetration enhancer compound. Adv Biosci Biotechnol 14(1): 1-17.
- Gharib G, Bütün İ, Muganlı Z, Kozalak G, Namlı İ, et al. (2022) Biomedical Applications of Microfluidic Devices: A Review. Biosensors (Basel) 12(11): 1023.
- Camargo IM, Saiki M, Vasconcellos MB, Avila DM (2001) Abrasiveness evaluation of silica and calcium carbonate used in the production of dentifrices. J Cosmetic Sci 52(3): 163-167.
- Majeed A, Grobler S, Moola M (2011) The pH of various tooth whitening products on the South African market. J South Afr Dental Assoc 66: 278-281.
- Sarembe S, Ufer C, Kiesow A, Limeback H, Meyer F (2023) Influence of the amount of toothpaste on cleaning efficacy: An in vitro Eur J Dent 17: 497-503.
- Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, et al. (2015) TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20(2): 107-126.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Pathology and Toxicology Research
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)